Received 8 April 2020; Revised 2 May 2020; Accepted 6 May 2020; Published 20 June 2020
Faten M. Ali Zainy, Aisha M. Turkustani, and Azza A. Shoukry, Synthesis, Characterization, Physicochemical Studies and Antimicrobial Evaluation of Mixed Ligand Complexes Involving Co(II) with 2,2′-Dipyridylamine and Dicarboxylic Acids, Journal of Transition Metal Complexes, 3 (2020), art246098. doi:10.32371/jtmc/246098
In the current research, the complex formation equilibria of 2,2′-dipyridylamine (DPA) with the metal ions Cu(II), Ni(II), Co(II), Mn(II), and Zn(II) are investigated potentiometrically. The relation between the stability constants of the formed complexes and the properties of the central metal ions was investigated. In addition, the solvent effect on the protonation of DPA and Co-DPA complex formation was also investigated and discussed. The formation of the ternary complexes Co(DPA)L (L = some selected dicarboxylic acids) was studied in aqueous solutions at 25 °C and 0.1 mol dm−3 ionic strength. Stability constants and stoichiometry are reported for the
complexes formed in solution. The results show that ternary complexes are formed by a simultaneous reaction mechanism. The speciation of all the complexes was resolved. The effect of chelate ring size of the dicarboxylic acid complexes on their stability constants was also examined. The concentration distribution of the complexes in solution was evaluated. The solid complexes [Co(DPA)L] (where L is oxalic, malonic, succinic or 1,1-cyclobutanedicarboxylic (CBDC) acid) have been synthesized and fully characterized with the help of elemental analyses, infrared spectra, magnetic and conductance measurements. Spectroscopic studies and magnetic measurements (Meff) suggest a tetrahedral geometry for Co(II)-complexes. The measured molar conductance values in DMSO indicate that the complexes are nonelectrolytic in nature. The isolated solid complexes have also been screened for their pharmacological activities against some selected bacteria and fungi. The activity data show that the complexes have a significant activity against Escherichia coli (gram negative) and Staphylococcus aureus (gram positive), as well as an antifungal activity against Aspergillus flavus and Candida albicans.
Co(II); 2,2′-dipyridylamine (DPA); dicarboxylic acids; potentiometry; stability constant; effect of solvent; biological activity
Metals have an esteemed place within medical biochemistry, although until recently this was restricted only to organic drugs. Recently, more research has been directed to the area of inorganic chemistry, which led to the developments in the treatment of cancer, diabetes, and ulcers as well as in the development of neurological, cardiovascular, and anti-inflammatory drugs. Metal coordination complexes have been widely studied for their antimicrobial [1,2] and anticancer properties [3], particularly those containing the late first row transition metals (i.e., cobalt (Co), nickel (Ni), copper (Cu), and zinc (Zn)) which are biologically relevant as they are associated with various biomolecules related to essential physiological activities [4]. Many drugs possess modified pharmacological and toxicological properties when administered in the form of metallic complexes. It was observed that the biological activity of these drugs increases on complexation with metal ion [5,6]. The factors affecting the stability of metal complexes, such as electrostatic, hydrophobic or steric interaction between the ligands, have been extensively studied based on the idea that these factors could play an important role in enzyme-metal ion-substrate systems [7].
Binary and ternary chelations occur commonly in biological fluids, as millions of potential ligands like amino acids, peptides or their derivatives or analogues, and heterocyclic N-bases are likely to compete for biologically important transition metal ions such as Cu(II), Ni(II), and Zn(II) found in vivo. These chelations, especially complexes that contain the two different types of bioligands (i.e., heteroaromatic nitrogen-base 2,2′-dipyridylamine (DPA) and dicarboxylic acids), may be considered as models for substratemetal ion-enzyme interactions and other metal ion mediated biochemical interactions.
Figure 1: The chemical structure of DPA.
From the biological point of view, Co(II) is one of the most important trace elements in the world of animals and humans. It is the key constituent of cobalamin, also known as vitamin B12, which is the primary biological reservoir of cobalt. Cobalamin plays a number of crucial roles in many biological functions as it is necessary for DNA synthesis, formation of red blood cells, maintenance of the nervous system, and the growth and development of children. Recently, it has been reported that some complexes of Co(II) with different ligands (e.g., cholic acids, Mannich bases, and mixed ligands) reduced significantly the viability and proliferation of cultured tumor cells and induced DNA damages in the treated cells. Moreover, coenzyme B12 features reactive C−Co, N−Co bonds, which participate in its reactions [8].
The mixed ligand complexes derived from the bioactive potent ligands containing N,O-donor binding sites with M(II) ions are used in a number of fields like biological, analytical, agricultural, industrial, and therapeutic applications [9,10]. Dicarboxylic metal complexes attract considerable attention in recent years because their interesting molecular topologies and crystal packing motifs along with potential applications are in many areas including gas storage, separation, catalysis, magnetism, optics as well as electrical conductivity [11,12]. Previous studies by Sigel [13] have revealed that certain ligand combinations lead to an increased stability, particularly if the mixed ligand complex is formed by a heteroaromatic N-base like 2,2′-bipyridine and an O-donor ligand-like malonate or pyrocatecholate. This enhanced stability is lost if the heteroaromatic N-base is replaced by an aliphatic amine like 1,2-ethylenediamine or an aromatic moiety like 1,2-diaminobenzene.
Therefore, this study becomes of paramount importance especially for researchers in the field of bioinorganic and medicinal chemistry and also can serve as a useful model for gaining understanding of enzyme-metal ion-drug complexes. In this investigation and as a continuation of our research program directed to investigate binary and ternary metal complexes involving biologically relevant metal ions, amino acids, amides, and DNA units [14,15,16,17,18,19,20], we report in this paper a quantitative study of the formation equilibria of binary complexes of DPA with Cu(II), Ni(II), Co(II), Mn(II), and Zn(II). We have also studied the effect of metal ion properties on the log K1 values of the binary metal complexes. The effects of dioxane as a solvent on both the protonation constants and the formation constants of Co-DPA binary complexes were discussed, in an effort to investigate complex formation equilibria in solvents of lower polarity that represent some biological microenvironments, or simulate to some degree the situation in active site cavities.
The solid mixed ligand complexes involving Co-DPA with some chosen dicarboxylic acids, such as malonic, oxalic, succinic, and 1,1-cyclobutanedicarboxylic (CBDC) acids, are synthesized and characterized by physicochemical methods. The isolated solid complexes were tested for their antimicrobial activities against some selected bacteria and fungi in the sense that testing the biological activities for such systems is needed for researchers working in various fields of biology, pharmacy, and medicine.
All the chemicals used in this study were of the analytical grade (AR). DPA was purchased from Sigma Aldrich, UK. The metal salts used (CuCl2·2H2O, NiCl2·6H2O, CoCl2·6H2O, ZnCl2·2H2O, and MnCl2·2H2O) are obtained from Sigma Aldrich. The dicarboxylic acids (oxalic, malonic, succinic, adipic, and CBDC acids) were provided by Sigma Aldrich with concentration of 0.01 mol dm−3. DPA was prepared in three equivalents (0.03 mol dm−3) of HNO3 acid. HNO3 was prepared by diluting concentrated ampoules (Riedel-de Haën, UK) and standardized against sodium carbonate. Dioxane was
provided by Sigma Aldrich. NaOH (titrant) was prepared and standardized against primary standard potassium hydrogen phthalate solution. The absence of carbonate in the titrant was periodically checked by pH potentiometry using the appropriate Gran function [21]. All solutions were prepared shortly before use in deionized water. The metal content of solutions was determined by complexometric EDTA titration [22].
The following procedure was used for the synthesis of the 1:1:1 ternary complexes. A minimum quantity of the chloride metal salt (500 mg, 2.10 mmol) dissolved in water was added slowly to the DPA (359 mg, 2.10 mmol) with continuous stirring. The dibasic acids (222 mg, 2.10 mmol for malonic), (302 mg, 2.1 mmol for CBDCA), (265 mg, 2.10 mmol for oxalic), and (248 mg, 2.10 mmol for succinic) were then added to the above mixture. Na2CO3 (222 mg, 2.10 mmol) was then added to neutralize the protons released. The mixture is stirred with heating for further 2 h and stored over night. The precipitated solid complexes were isolated by vacuum filtration and washed three times with water, ethanol, and finally with diethyl ether and dried on vacuum over silica gel. The yield ranged from 80% to 90%. The complexes were subjected to elemental and spectroscopic analysis. The elemental analyses were conducted using the Perkin-Elmer 2400 Analyzer and the results were found to be in good agreement (±0.3%) with the calculated values.
pH-metric measurements were performed by using a Metrohm 686 Titroprocessor equipped with 665 dosimate. The titration cell used for the titration is a double wall cell of 50 mL capacity equipped with a magnetic stirrer. The cell solution was continuously stirred during the titration using magnetic stirring system. The cell is covered with a calibration lid and contains four holes for Metrohm glass electrode, glass tubing for nitrogen injection (degassing), thermometric probe, and a plastic tube for alkaline solution. Before filling of a tube with alkali solution, the tube was washed several times with distilled water and then washed with alkali solution at least three times. Also, the air bubbles were avoided to leak in the tube in order to get accurate results for the measured volumes. The titroprocessor and the glass electrode were calibrated with standard buffer solutions of both phosphate mixture of KH2PO4 and Na2HPO4 (pH 6.865) and potassium hydrogen phthalate solution (pH 4.008) [23]. The titration reaction was performed using standard solution of 0.05 mol dm−3 carbonate free sodium hydroxide solution in presence of purified N2 atmosphere. The pH meter readings were converted into hydrogen ion concentration as given in the literature using Van Uitert and Hass equation [24]. All potentiometric titrations were carried out at 25 ± 0.05 °C. The temperature of all solutions was maintained at 25 ± 0.05 °C by circulation of thermostated water through the outer jacket of the cell. All titrations were performed in triplicate. Estimated precision was ±0.1 mV and ±0.003 mL for the EMF and titrant readings, respectively.
As is well known, pH-meters read −log10 αH+ (pH) whereas the potentiometric method we used for the calculation of stability constants requires −log10 [H+] (p[H]). The pH-meter readings (B) recorded in dioxane-water solutions was converted to hydrogen ion concentrations [H+] by using the widely used Van Uitert and Hass equation [24]:
|
|
(1) |
where log10UH is the correction factor for the solvent composition and ionic strength for which B is determined. Values of pKw in dioxane-water mixtures were determined as described previously [24,25]. For this purpose, various amounts of standard NaOH solution were added to a solution containing 0.1 mol dm−3 NaNO3. The values of [OH−] were calculated from the amount of base added; [OH+] was calculated from the pH. The products of [OH−] and [H+] were used to calculate the pKw values described above. The mean values obtained in this way at 25 °C for log10 [H+][OH−] are pKw = 14.17, 14.60, 15.12, and 15.49 for 12.5%, 25%, 37.5% and 50% dioxane-water solutions, respectively. The values were found to be consistent with literature data [26,27,28].
The protonation constants of the ligands in the protonated form were determined potentiometrically by titrating the ligand (40 cm3) solution (1.25 × 10−3 mol dm−3) of constant ionic strength 0.1 mol dm−3 (adjusted with NaNO3). The stability constant of the M(II)(DPA) complexes was determined by titrating 40 cm3 of a solution mixture of M(II) (1.25 × 10−3 mol dm−3), DPA ligand (2.5 × 10−3 mol dm−3) in concentration ratio 1:2 and NaNO3 (0.1 mol dm−3). The conditions of measurements of the ternary complexes were the same as for the binary ones, but the solutions contained equivalent amounts of DPA, Co(II), and dicarboxylic acid ligands (L) in concentration ratio 1:1:1.
The microchemical analysis of the separated solid complexes for C, H, and N was performed in the Microanalytical Center, Cairo University. The analyses were performed twice to check the accuracy of the analytical data. IR spectra were measured on a 80486-pc FTIR Shimadzu spectrophotometer using KBr pellets. UV-visible spectra of freshly prepared solutions of the complexes were recorded at room temperature using a Shimadzu UV-1800 recording spectrophotometer. The molar conductance of the complexes was measured in 1.0 × 10−3 mol dm−3 DMSO solution at 25 °C using a Jenway 4510 conductivity meter.
The calculations were obtained from Ca. 100 data points in each titration using the least-squares computer program MINIQUAD-75 [29]. The stoichiometries and stability constants of the complexes formed were determined by trying various possible composition models. The model selected gave the best statistical fit and was chemically consistent with the titration data without showing any systematic bias in residuals [29]. The fitted model was tested by comparing the experimental titration data point and the theoretical (simulated) curve calculated from the values of the acid dissociation constants of the ligand and the formation constant of the corresponding complex, as given in Table 1. The complexation equilibria were elucidated from the concentration distribution curves obtained with the program SPECIES [30] under the experimental condition used.

Table 1: Formation constants of the binary complexes of DPA at 25 °C and I = 0.1 M ionic strength.
Disk diffusion susceptibility method [15] in accordance with National Committee for Clinical Laboratory Standards (NCCLS) guidelines was used for initial screening of compounds for antibacterial activity against gram-positive (G+) bacteria Staphylococcus aureus, gram-negative (G−) bacteria Escherichia coli and also for their antifungal activity against Candida albicans and Aspergillus flavus described in British Pharmacopoeia (2000). Nutrient agar was melted at 45.00 °C, and inoculated with the cell suspension (1:100) bacteria or yeast. The flask was shaken well and poured into a Petri-dish (15 cm in diameter). Filter paper discs (6 mm) Whatman No. 2 were thoroughly moistened with antibiotics (50 g). The treated discs were aseptically transferred and placed on the surface of the inoculated plates and kept in a refrigerator for 1 h to permit diffusion of antimicrobial substances. The plates were incubated at 7.00 °C, for 24 h in the case of bacteria and at 28.00 °C, for 48 h in the case of fungi. The zones of inhibition were measured in mm. The mean values of inhibition were calculated from triple reading in each test [15]. For studying the antimicrobial properties of drug complexes, the following media were used (weights are given in gram per one-liter medium): (1). Nutrient agar medium (pH = 7.4): it consists of beef extract (1 g), yeast extract (2 g), peptone (5 g), sodium chloride (5 g), agar agar (15 g), and distilled water (100 cm3); (2). Sabouraud dextrose agar (SDA) medium (pH = 5.6): it consists of peptone (10 g), dextrose (20 g), agar agar (15 g), and distilled water (100 cm3). Moreover, some commercialized antibiotics were evaluated for their antibacterial activity and a comparison with the synthesized complexes present in this investigation was drawn. The results are expressed as zone of inhibition in millimeters (mm). Experiments were done in triplicate and an average standard deviation of < 2 was observed.
Before calculation of the stability constants of metal complexes, the acid dissociation constant of the ligand should be first determined. The cumulative (overall) formation constant βpqr of a species is defined on the basis of the following equilibrium (where charges on individual species are omitted for simplicity):
|
|
(2) |
|
|
(3) |
If there is no metal complex formation (p = 0), then only protonation of the ligand occurs. The ligand is prepared in three equivalents HNO3 solution. Analysis of the potentiometric data of DPA in the triprotonated form yields two pKa values corresponding to the protonated amino groups (Table 1). These results are in agreement with previous investigation [15]:
|
|
(4) |
|
|
(5) |
Each metal ion with the ligand was titrated with NaOH. Comparing the titration curve of the free ligand with those of the complexed ligand indicated that addition of the metal ions to the free ligand shifts the curve to lower pHs. In other words, the curves of the complexes are situated at lower pHs than the free ligand curve as shown in Figure 2. This can be explained simply as a result of proton release from the coordinated ligand, which implies formation of complexes through release of hydrogen ion upon complex formation. The complex formation equilibria of the metal complexes are characterized by fitting the potentiometric data to various models. All feasible theoretical models were compared with those experimentally obtained. The equilibrium patterns were chosen so as to lie between the observed and the calculated data applying accurate statistical analysis involving the sum of squares of residuals. The whole titration data obtained was fitted with different composition models. The best model was found to be consistent with the formation of the complexes with stoichiometric coefficient 110 together with the protonated form in case of Mn(II) ions (Table 1). The validity of the complex formation model was tested by comparing the potentiometric titration data with the theoretically simulated curve obtained from the protonation constants of the ligand and the formation constants of the corresponding complex species.

Figure 2: Potentiometric titration curve of the [Co-DPA] system.
Estimation of the concentration distribution of the various species in solution provides a useful picture of metal ion binding. The main features observed in the species distribution plots in these systems are shown in the speciation diagram obtained for Mn(II)-, Cu(II)-, and Co(II)-DPA complexes; as shown in Figure 3. The protonated complex for Mn(II) prevails with formation degree of 90% at pH ≈ 4.5. The 110 complex species starts to form at pH ≈ 4 and its concentration increases with increase of pH. Cu(II) and Co(II) also form 1:1 complexes with DPA with the stoichiometry 110, with formation degree of 55% and 45% at pH ≈ 4 and 6.5, respectively.
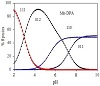
Figure 3: Concentration distribution of various species as a function of pH in the [M(II)-DPA] system; M(II) = Mn, Cu, Co.
It is necessary to correlate the stability constants of metal complexes with the characteristic properties of the metal ions, in an effort to explain why a given ligand prefers binding to one metal rather than another, and to give information about the nature of chemical bonding in complexes and make possible the estimation of unknown stability constants. Here we have discussed the stability constants of the binary metal complexes (M(II)-L) and the ionic radius, the ionization energy, the atomic number, and the electronegativity of the selected metal ions. The formation constants of MII-complexes of bivalent 3D transition metal ions with DPA, as given in Table 1, are in the following order: Mn2+(logβ110 = 4.01) < Co2+(logβ110 = 4.55) < Ni2+(logβ110 = 6.88) < Cu2+(logβ110 = 7.65) > Zn2+(logβ110 = 5.87), which is in accordance with Irving-Williams order [16,32,33]. The correlation between the logKML and the reciprocal ionic radii (1/r) of the studied bivalent transition metal ions was found to be almost linear (Figure 4(a)). Also, a good linear correlation has been obtained between logKML and the electronegativities of the metal ions under study (Figure 4(b)). This in accordance with the fact that increasing electronegativity of the metal ions (Zn2+(1.65) < Co2+(1.88) < Ni2+(1.91) < Cu2+(2.0)) (Table S1) will decrease the electronegativity difference between the metal atom and the donor atom of the ligand. Thus, the metal ligand bond would have more covalent character, which may lead to greater stability of the metal chelates. A good linear relationship has been obtained between logKML and the second ionization potential of the bivalent metal ions under study (Figure 4(c)). In general, it is noted that the stability constant of the Cu2+ complex is quite large compared to the other metals. The ligand field will give Cu2+ some extra stabilization due to tetragonal distortion of the octahedral symmetry. The Cu(II) complex will be further stabilized due to the Jahn-Teller effect [34,35]. Thus, logK value for the Cu2+-complex deviates significantly when logK values of metal chelates are plotted against properties of the metal ions.
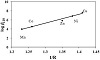
Figure 4: Effect of metal ion properties on the stability constant of DPA-complexes.

Table S1: Atomic number, ionic radius, electronegativity and ionization potential of the investigated bivalent metal iond.
Mostly, the physical constants of bioligands are studied in aqueous media. Despite this, little is known about the chemistry of biomolecules in mixtures of organic solvents and water. Determination of the protonation constants of bioactive ligands and the stability constants of their metal complexes in various media is important for complete understanding of the physicochemical behavior of such molecules. It is well established that the “effective” or “equivalent solution” dielectric constants in protein or active site cavities of enzymes are small compared to those in bulk water [14,36]. Estimates for the dielectric constants in such locations range from 30 to 70 [36]. Hence by using aqueous solutions containing ≈ 10–50% dioxane, one may expect to simulate to some degree the situation in active site cavities. This allows extrapolating the data into physiological conditions.
Careful examination of media effects on the equilibrium constants (Table 2) reveals the following features.
(1) pKa of DPA increases linearly with increasing the percentage of organic solvent in the medium (Figure 5). This may be correlated with the ability of a solvent of relatively low dielectric constant to increase the electrostatic forces between the proton and the ligand and, consequently, the pKa value increases.
(2) The stability constant (log10K1) of the Co(II)-DPA complex increases with the increase of dioxane concentration (Figure 5). This can be interpreted in terms of an electrostatic model. In general, lowering of the dielectric constant of a medium (by increasing dioxane content) favors the interaction between the Co(II) ion and DPA and, consequently, the stability constant of the complex increases.
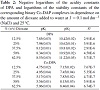
Table 2: Negative logarithms of the acidity constants of DPA and logarithms of the stability constants of the corresponding binary Co-DAP complexes in dependence on the amount of dioxane added to water at I = 0.1 mol dm−3 (NaCl) and 25 °C.

Figure 5: Effect of dioxane on the protonation constant of DPA and the formation constant of Co-DPA complexes. Curves (1) and (2) correspond to the dissociation of the DAP ligand, log K1 corresponds to 110.
In the Co(DPA)-dicarboxylic acid system, computer analysis of the pH titration data showed the presence of the 1:1:1 species and its protonated form. The results in Table 3 show that the formation constant of the 1:1:1 complexes involving the formation of five- and six-membered chelate rings may be attributed more to the higher stability of the five- and six-membered rings, known to be energetically favored, than the seven- and eight-membered rings. It is interesting to note that CBDC acid has a higher stability constant than malonic acid, although they both form six-membered chelate rings. This may be due to the higher pKa values of CBDC acid in comparison with the pKa values of malonic acid. The concentration distribution diagram of the [Co-DPA-CBDC acid] system (Figure 6) shows that the main species in the physiological pH range is the ring-closed form, 1110, which reaches a maximum concentration of ≈ 78% around pH 7. The interest in this high stability of the [Co-DPA-CBDC acid] complex at the physiological pH range is that the sugar group of DNA may undergo stacking interactions with the cyclobutane ring. Such interactions could favor reactions with DNA. The latter effect is similar to that reported for carboplatin, where stacking interactions between the cyclobutane ring of CBDC acid and the sugar group of DNA occur and form part of the increased antitumor activity [37].

Table 3: Stability constants of the ternary species in the CoII-DPA-dibasic acids and proton-association constants and their binary stability constants.

Figure 6: Concentration distribution of various species as a function of pH of the [Co(DPA)(CBDC acid)] system, at a concentration of 1.25 mL mol.L−1 for each.
All the synthesized complexes are air-stable, nonhygroscopic, high melting, and insoluble in H2O, but soluble in coordinating solvents such as DMF and DMSO. The formulation of these complexes is based on the elemental analysis, IR, UV-vis, magnetic and conductance measurements. The elemental analysis of the complexes (Table 4) revealed the presence of water molecules. All have the general composition [Co(DPA)L]nH2O (where L = Ox., Mal., Suc. or CBDC acid; n = 2 or 1). The complexes are nonionic as revealed by their low conductance values. The study of the spectral and magnetic properties of the solid complexes provides information that can throw considerable light on their geometry. Careful examination of the recorded spectra of the different synthesized ternary complexes reveals the following features.

Table 4: Microanalytical data, magnetic moment, molar conductance and electronic spectral data of the prepared ternary complexes.
IR spectra of the complexes exhibit strong NH absorption bands, being unchanged compared to their position in the ligand, indicating that the NH group of DPA is not involved in coordination. The IR spectra of the formed ternary cobalt complexes reveal the presence of water molecules, as indicated by a broad band of ν(O−H) at 3,420–3,450 cm−1 and two weaker bands at approximately 899–911 cm−1 and 685–690 cm−1, respectively [6,38], which is in accordance with the results of elemental analyses. It was reported that [39] coordinated water exhibit frequencies at 825 cm−1, 575 cm−1, and 500 cm−1. The absence of spectral bands in these regions indicates that the water molecules are not coordinated but are present as lattice water. The band in the region 1,625–1,610 cm−1, that is characteristic of the (C=N) stretching mode [40], is shifted towards lower frequencies in the spectra of the cobalt complexes 1,609–1,590 cm−1 indicating the involvement of the nitrogen in chelation with the cobalt ion [41]. The pyridyl ring vibration bands at 1,578–1,608 cm−1 and 765–779 cm−1, respectively, indicate that the nitrogen atoms of the pyridyl ring of the ligands donate a pair of electrons each to the central metal forming coordinate covalent bond [42]. This is further supported by the appearance of a new medium intensity band in the region 480–520 cm−1 assignable to ν(M−N) vibration [43]. The bands in the 2,923–2,530 cm−1 region (medium to weak) can be assigned to C−H stretching vibrations in both the primary and secondary ligands. The bands in the 1,619–1,603 cm−1 region in all the prepared complexes are typical of coordinated carboxylate group stretching [44]. This is based on the fact that the unionized and uncoordinated COO stretching band occurs at 1,750–1,700 cm−1 whereas the ionized and coordinated COO stretching band appears at 1,650–1,590 cm−1. It was reported previously that the magnitude of the Δν[Δν = νasy(COO−) − νsym(COO−)] can be correlated with the coordination modes of the carboxylate anion [45,46]. The calculated values of Δν for each of the studied complexes were found to be > 200 cm−1, reflecting the monodentate coordination of each carboxylate group. Therefore, the dicarboxylic acids act as bidentate
O−, O− ligands and coordinate to the Co(II) metal via two monodentate carboxylate groups. The band appearing in the 1,400–1,391 cm−1 region could be attributed to the symmetric stretching vibration of the coordinated carboxylates [44].
Further insight into the structures of the isolated solid complexes is proposed on the basis of the spectral and magnetic studies. The spectra of all of the complexes contain
an absorption band in the 15,822–16,260 cm−1 range that may be assigned to a d–d transition of metal ions [47]. The Co(II) complexes show bands at 16,168 cm−1, 16,210 cm−1,
15,844 cm−1, and 15,723 cm−1 for the CBDC, malonate, oxalate, and succinate complexes, respectively, (Table 4). These bands are probably due to the 4A2(F) → 4T1(P) transition of tetrahedral geometry [48]. Charge transfer transition bands are also appearing for all the studied Co(II) complexes at the range of ca. 23,070–23,350 cm−1 as reported previously for similar systems [49,50].
The observed values of magnetic moments for complexes are generally diagnostic of the coordination geometry about the metal ion. The calculated magnetic moment of the synthesized complexes is given in Table 4. All the values are in the range of 4.1–4.5 B.M, which are in consistence with a tetrahedral geometry for the Co(II) ion as reported previously [51,52].
The synthesized ternary complexes were dissolved in DMSO and the molar conductivities of 10 mol dm−3 of their solutions were measured at 25 °C. The values obtained are given in Table 4. The molar conductivity values (Λ M) of the Co(II) complexes were found to be between 15.9 Ω−1 cm2 mol−1 and 20.8 Ω−1 cm2 mol−1. The low values indicate that all Co(II) complexes are nonelectrolytes. This is in accordance with the fact that the conductivity values for a nonelectrolyte are below 50 Ω−1 cm2 mol−1 in DMSO solution [53,54].
From all of the above observations and according to the results reported in this paper based on the analytical data, IR, molar conductivity, spectral (solid reflectance) and magnetic measurements, the structure of these mixed-ligand complexes is tetrahedral. This indicates that the DPA ligand behaves as a bidentate chelating ligand through the two pyridine rings while the dicarboxylic acids are coordinated via the two oxygen atoms of carboxylate groups.
Metal chelates were well known to have an enhanced antimicrobial and biological activity. It has been found that the Cu(II) complex of DPA catalysis the hydrolysis of methyl acetate, simple amides, and triesters of phosphoric acids [55]. These systems are considered as very bioactive models for metalloenzyme substrate interaction. In this study, we had screened the biological potential of the synthesized complexes in vivo against different species of bacteria and fungi. In the process of testing the antimicrobial activity of these compounds, we have used more than one test organism in order to increase the chance of detecting antibiotic principles in the tested materials. The organisms used in the present investigations include one G+ (S. aureus) as well as one G− (E. coli) bacteria. All of the tested compounds show a remarkable pharmacological activity against different bacterial strains, that is, G+ and G− bacteria. The disc diffusion method was used to evaluate the antibacterial activity of the synthesized ternary complexes. The medium used for growing the culture was nutrient agar. The data are listed in Table 5. The increase in biological activity of the metal chelates may be attributed to the effect of the metal ion on the normal cell process. The cell toxicity is increased and this could be explained in the light of Tweedy’s chelation theory [56]. The polarity of the metal ion is considerably reduced upon chelation because of partial sharing of metal positive charge with the donor ligand, and the possible π-electron delocalization that occurs within the whole chelate ring upon coordination. This chelation could enhance the lipophilic character of the central metal atom and accordingly the hydrophobic character and liposolubility of the complex would increase, favoring the process of permeation through the lipid layers of the cell membrane. This would increase the rate of uptake and entrance of the complexes into the cell membrane, thus the antimicrobial activity of the testing compounds. Accordingly, the antimicrobial activity of the complexes present in this investigation can be referred to the increase of their lipophilic character which in turn deactivates enzymes responsible for respiration processes and probably other cellular enzymes, which play a vital role in various metabolic pathways of the tested microorganisms. On comparing the biological activity of the synthesized complexes with the standards tetracycline (antibacterial agent) and amphotericin B (antifungal agent), the following results are obtained:
(a) using G− E. coli and G+ S. aureus, we discovered that the biological activity of the CBDC acid complex is
higher than that of the malonate, oxalate, and succinate complexes and slightly lower than that of tetracycline standard;
(b) all complexes were found to be more active against G+ than G− bacteria.

Table 5: The antibacterial and antifungal activity of the synthesized metal complexes.
The antifungal activities of the synthesized complexes were tested against two fungi (A. flavus and C. albicans) test organisms in vitro using disc-diffusion method. The amphotericin B was used as a standard drug. The susceptibility of the selected strains of fungi towards the synthesized complexes was judged by measuring the diameter of the formed
zone of inhibition. The results are given in Table 5. Metal
ions are adsorbed on the cell walls of the microorganisms, disturbing the respiration processes of the cells and thus blocking the protein synthesis that is required for further growth of the organisms. Hence, metal ions are essential for the growth-inhibitory effects. According to Overtone’s concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid-soluble materials, so lipophilicity is an important factor controlling the antifungal activity. Upon chelation, the polarity of the metal ion will be reduced due to the overlap of the ligand orbitals and partial sharing of the positive charge of the metal ion with donor groups. This increased lipophilicity facilitates the penetration of the complexes into lipid membranes, further restricting proliferation of the microorganisms. The data in Table 5 shows the following:
(c) using A. flavus fungus: the antifungal activity of the CBDC acid, the oxalate and the succinate complexes are higher than that of the standard antifungal agent amphotericin B; whereas, the antifungal activity of the malonate complex is slightly lower than that of the standard;
(d) using C. albicans fungus: the biological activity of malonate complex is found to be higher than the other complexes, and all have lower values than that of the standard. The importance of this lies in the fact that these complexes could be applied fairly in the treatment of some common diseases caused by E. coli, for example, Septicaemia, Gastroenteritis, Urinary tract infections, and hospital acquired infections [49] or any other infections caused by any of these particular strains.
The authors declare that they have no conflict of interest.
Copyright © 2020 Faten M. Ali Zainy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.